How Many Valence Electrons Are in Hafnium
Electronic configuration of Hafnium Xe 4f 14 5d 2 6s 2. Classified as a transition metal Hafnium is a solid at room temperature.

Hf Hafnium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
Ok but how many valence electrons does an atom of Hafnium have.

. 72 Number of Neutrons. Total number of valence electrons in an HF molecule 1 7 8. 2506 K 2233 C 4051 F Boiling point.
One of the oldest procedures for the determination of zirconium and hafnium is based upon precipitation as phosphate from dilute sulfuric acid solution. The ground state electron configuration of ground state gaseous neutral hafnium is Xe. For main group elements ie s-block and p-block elements the valence.
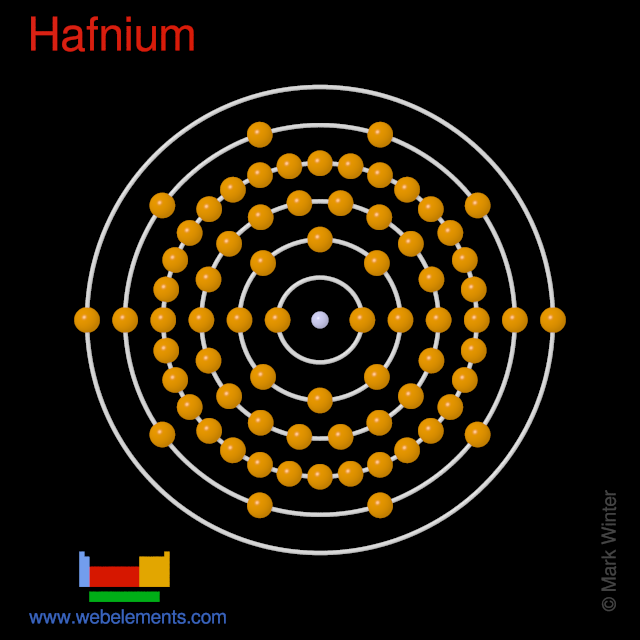
We can check the atomic number from the periodic table to confirm the valence electron number. Electron configuration of Hafnium is Xe 4f14 5d2 6s2. Hafnium atoms have 72 electrons and the shell structure is 281832102.
Diagram of the nuclear composition electron configuration chemical data and valence orbitals of an atom of hafnium-178 atomic number. Zirconium and hafnium are difficult to determine by atomic absorption spectroscopy AAS. 17849 amu Melting Point.
Electron Configuration and Oxidation States of Hafnium. The nucleus consists of 72 protons red and 106 neutrons orange. 4876 K 4603 C 8317 F Density near rt 1331 gcm 3.
Atoms of the noble gases have 8 valence electrons except for helium which has 2. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. Hexagonal Density 293 K.
Number of Neutrons most commonstable nuclide. 21500 C 242315 K 39020 F Boiling Point. Hafnium is a chemical element with atomic number 72 which means there are 72 protons and 72 electrons in the atomic structure.
Atomic number of Hafnium or Protons in Hafnium. 54000 C 567315 K 97520 F Number of ProtonsElectrons. The chemical symbol for Hafnium is Hf.
Hydrogen belongs to period 1 and has 1 valence electron whereas Fluorine belongs to period 17group of halogens and therefore has 7 valence electrons. Atomic radius of Hafnium. Hafnium is a lustrous silvery gray tetravalent transition metal hafnium chemically resembles zirconium and is found in many zirconium minerals.
Hafnium is a lustrous silvery gray tetravalent transition metal hafnium chemically resembles zirconium and is found in many zirconium minerals. Hafnium may be found in column 4 of a wide form periodic table. Number of Electrons with no charge.
The chemical symbol for Hafnium is Hf. The number of electrons in an electrically-neutral atom is the same as the number of protons in. Hafnium Overview Hafnium Valence Electrons 4 Atomic Number 72.
Now lets check the facts about Hafnium. 2 8 18 32 10 2. A valence electron is an outer shell electron and may participate in the formation of a chemical bond.
Atoms with 8 valence electrons or 2 in the case of helium are stable. 132 gcm 3 Color. This indicates that it has four valence.
How many valence electrons are in the outer shell of Hafnium with an atomic number of 72. Below are their quantum numbers N - energy L - angular momentum M - magnetic moment S - spin Orbital. The chemical symbol for Hafnium is Hf.
2573 JmolK Vapor pressure. In the case of Hafnium the valence electrons is 4. Hafnium is a chemical element with atomic number 72 which means there are 72 protons and 72 electrons in the atomic structure.
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. The number of valence electrons in a Hafnium atom - 4. When liquid at mp 12 gcm 3.
72 electrons white successively occupy available electron shells rings. In the below periodic table you can see the trend of Valence Electrons. Atomic mass of Hafnium.
Electrons per Energy Level. 212 picometers van der Waals radius. 72 the most common isotope of this element.
Transition Metal Crystal Structure. Electrons arrangement in Hafnium or Bohr model of Hafnium. Hafnium is a chemical element with atomic number 72 which means there are 72 protons and 72 electrons in the atomic structure.
P G JEFFERY D HUTCHISON in Chemical Methods of Rock Analysis Third Edition 1981. For facts physical properties chemical properties structure and atomic properties of the specific element click. They are unlikely to gain or lose electrons or to share electrons with other atoms.
Chemical Properties of Hafnium. 5d 6 6s 2 Electron Dot Model. 2 8 18 32 10 2.
Possible oxidation states are 4. Hafnium is a chemical element with symbol Hf and atomic number 72. Unabbreviated electronic configuration of neutral Hafnium.
Therefore the number of electrons in neutral atom of Hafnium is 72. Periodic Table of Elements with Valence Electrons Trends. 6s2 and the term symbol is 3F2.
For atoms with many electrons this notation can become lengthy and so an abbreviated notation is usedThis is important as it is the Valence electrons 4f14 5d2 6s2 electrons in the outermost shell that determine the chemical properties of the element.

Hafnium Atomic Structure Stock Image C018 3753 Science Photo Library
Webelements Periodic Table Hafnium Properties Of Free Atoms


Comments
Post a Comment